The periodic table is an arrangement of the elements in order of their atomic weights. There are 103 elements represented on the modern table which is based on Mendeleev's original one.
The periodic table is an arrangement of the elements in order of their atomic weights. There are 103 elements represented on the modern table which is based on Mendeleev's original one.

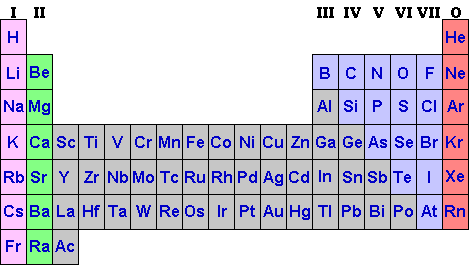
 Although historically, a number of scientists worked on listing the natural elements, it was Dimitri Mendeleev, a Russian chemist, who brought order to the list of elements. By 1850, 59 of the 92 natural elements had been discovered. Mendeleev looked at similarities in the chemical reactions of elements they knew about, and patterns in their physical properties such as melting and boiling points and densities. He grouped elements that were similar so that they fell together in a vertical column. Mendeleev had enough foresight to leave gaps in his organisation table to allow for the possible discovery of new elements. His periodic table of the elements was first published in 1869. Some would argue that it is the most "amazingly compact store of information ever produced. With a copy of the periodic table in front of you and some knowlegde of how to put it together, you have thousands of facts at you fingertips".
Although historically, a number of scientists worked on listing the natural elements, it was Dimitri Mendeleev, a Russian chemist, who brought order to the list of elements. By 1850, 59 of the 92 natural elements had been discovered. Mendeleev looked at similarities in the chemical reactions of elements they knew about, and patterns in their physical properties such as melting and boiling points and densities. He grouped elements that were similar so that they fell together in a vertical column. Mendeleev had enough foresight to leave gaps in his organisation table to allow for the possible discovery of new elements. His periodic table of the elements was first published in 1869. Some would argue that it is the most "amazingly compact store of information ever produced. With a copy of the periodic table in front of you and some knowlegde of how to put it together, you have thousands of facts at you fingertips".
There are eight of Mendeleev's vertical columns or groups in the table and a group of elements in the middle called transition elements. The middle group do not concern us here. Elements in the same group have the same number of electrons in their outer shell, denoted by the roman numeral at the top of the group. Therefore they react in similar ways as they all strive to lose, gain or share the same number of electrons. As we shall see later, chemical conversations and relationships are determined by the electron number in the outer shell.
Take a look at the periodic table above and notice the group 0. These are called the Noble or Inert elements because their outer shell is full of electrons. They are very stable and tend to be unreactive and really couldn't be bothered associating with other elements. So they don't. Why should they? (Life could not be any sweeter) Their lives couldn't possibly be any better by such associations. A bit snobby, really. As it happens, other elements would like to be like the Noble gases too and will react in a way so as to achieve this. I suppose we all have allusions to royalty, even elements!
For example, find Sodium (Na), atomic number 11. From the table, you can see that to get to the Noble gas state, Sodium can either "move left" one place and wrap around to become Noble or "move right" seven places to get to the same spot. If you were in this predicament, which way would you go? The shorter distance of course! If Sodium can just shrug off one electron, ("move left") it will have made the big time, become Noble and have a full outer shell of electrons. So, Sodium and its cohorts will give away one electron (group 1) at the drop of the hat, i.e. they are very reactive elements and react in a similar fashion to achieve nobility.
|