The Preparation of Oxygen in the Laboratory
You will need
: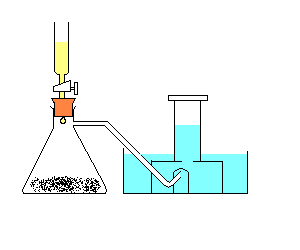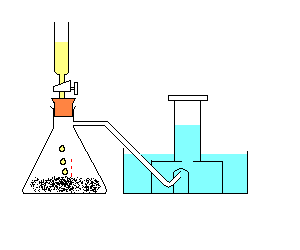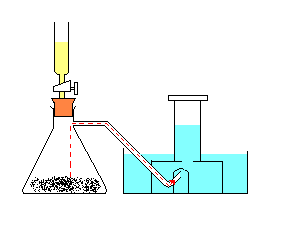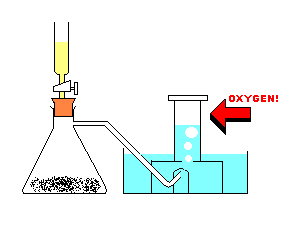
Procedure
:Note: The hydrogen peroxide acts as a catalyst. A catalyst is used to speed up a chemical reaction.
|
The Preparation of Oxygen in the Laboratory |
|
|
You will need : |
|
|
|
|
|
Procedure : |
|
|
Note: The hydrogen peroxide acts as a catalyst. A catalyst is used to speed up a chemical reaction. |
|