 A
battery is a store of chemical energy. The stored energy turns into
electrical energy when the battery is used. Inside the casting, different
chemicals are stored which react together to create an electrical current. A
battery is a store of chemical energy. The stored energy turns into
electrical energy when the battery is used. Inside the casting, different
chemicals are stored which react together to create an electrical current.
If you look at a battery you will
notice that it has two terminals. One of them will be marked + (positive)
and the other will be marked - (negative). On an AA battery the terminals
are at each end of the battery but on some bigger batteries both terminals
are at the same end.
 Electrons
collect on the negative terminal of the battery. If you connect a wire
between the positive and negative terminals the electrons will flow
from the negative terminal to the positive terminals as fast as they
can. This will wear out your battery very quickly. This is also very
dangerous especially with big batteries. This is because the electrons
flow so quickly they start to heat up the battery. Usually you would
put something like a light bulb or a motor to it. Electrons
collect on the negative terminal of the battery. If you connect a wire
between the positive and negative terminals the electrons will flow
from the negative terminal to the positive terminals as fast as they
can. This will wear out your battery very quickly. This is also very
dangerous especially with big batteries. This is because the electrons
flow so quickly they start to heat up the battery. Usually you would
put something like a light bulb or a motor to it.
Inside a battery a chemical reaction is what makes the battery work.
The electrons must flow from the negative terminal for the reaction
to work. The electrons flow through the wire, into your item and back
to the battery. This is called a circuit. Unless electrons are flowing
from the negative terminal the reaction will not work. That is why you
can leave a battery on a shelf and it will not loose its power.
You can make your own battery
by using very basic things. You need one copper-coated and one zinc-coated
nail ,some thin insulated wire ( about 50cm), a compass and a container
of salty water or watered-down vinegar.
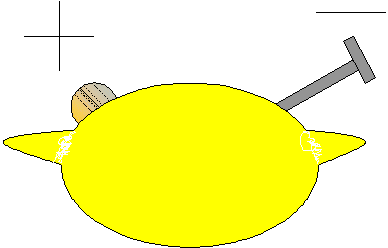
One of the simplest
batteries you can make is out of a lemon, a copper coin and a galvanised
nail (galvanised nails are coated in zinc). To make the battery, insert
the coin into a slot in one side of the lemon. Press the nail into the
other side, taking care to make sure that the nail and coin do not touch
each other. These batteries only create a voltage of .906 volts but
you can wire two or more of them together to produce more volts.
The first battery was created by Alessandra Volta in 1800. To find out
more about Volta, please click
here.
C. K. and A.
H.
Electricity
Homepage
|


 A
battery is a store of chemical energy. The stored energy turns into
electrical energy when the battery is used. Inside the casting, different
chemicals are stored which react together to create an electrical current.
A
battery is a store of chemical energy. The stored energy turns into
electrical energy when the battery is used. Inside the casting, different
chemicals are stored which react together to create an electrical current.
 Electrons
collect on the negative terminal of the battery. If you connect a wire
between the positive and negative terminals the electrons will flow
from the negative terminal to the positive terminals as fast as they
can. This will wear out your battery very quickly. This is also very
dangerous especially with big batteries. This is because the electrons
flow so quickly they start to heat up the battery. Usually you would
put something like a light bulb or a motor to it.
Electrons
collect on the negative terminal of the battery. If you connect a wire
between the positive and negative terminals the electrons will flow
from the negative terminal to the positive terminals as fast as they
can. This will wear out your battery very quickly. This is also very
dangerous especially with big batteries. This is because the electrons
flow so quickly they start to heat up the battery. Usually you would
put something like a light bulb or a motor to it.