Thermionic Emission.
Thomas Edison, an American physicist, was the first to observe a phenomenon, since known as thermionic emission or the thermionic effect. Thermionic emission is the emission of electrons from a hot metal.
In 1883, while experimenting with an electric lamp which contained a metal plate near the filament and connected to it via a galvanometer and battery, Edison found that a current flowed through the galvano- meter when the plate was positive with respect to the filament. However, when the plate was negative with respect to the filament the galvanometer registered no current. Edison could not explain this phenomenon-then known as the Edison effect-and he - disregarded it.
In 1902, Sir Owen W. Richardson ( 1879-1959), an English physicist, explained the Edison effect in terms of electrons evaporating from the hot metal surface of the filament. When the metal plate was positive it attracted these electrons to it, and the flow of electrons constituted a current. When the metal plate was negative with respect to the filament it repelled the electrons away from it.
Since there was no flow of electrons to the plate the galvano- meter did not register a current. The phenomenon then became known as thermionic emission.
Richardson was awarded the Nobel prize for physics in 1928 for his work on thermionic phenomena.
Thermionic Emission is the
emission of electrons
From the surface of a hot metal.
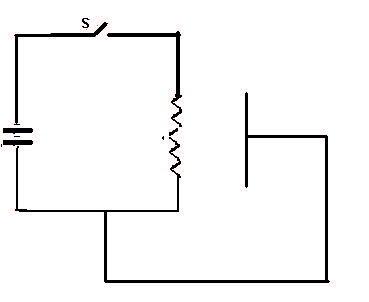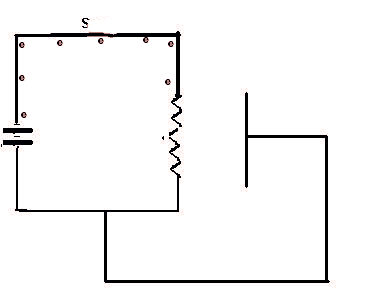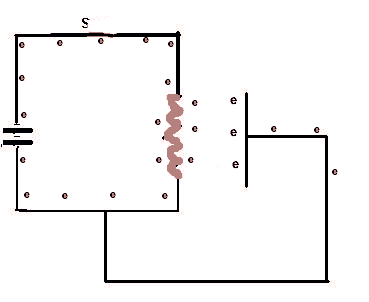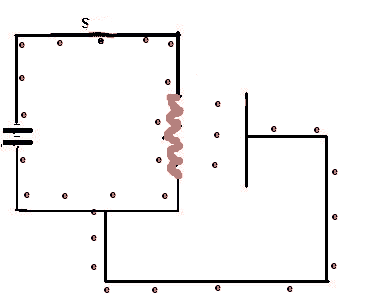
When the switch S is closed, a complete circuit if formed and electrons (e), electric current, begins to flow. When they reach the wire filament it heats up and then emitts electrons which will flow around the external circuit. This sequence is repeated 5 times.
How is the "evaporation" of electrons from the surface of a metal explained?
In the closely packed atoms of the metal. the electron in the valency shell (the outer shell) are not strongly attracted by the nucleus. As a result they become free of any particular atom and exist as an electron "gas" within the metal itself. The electrons are held in the metal by the attraction of the positive ions of the metal.
However, electrons near the surface can escape from the metal if they have enough kinetic energy to overcome the attractive forces . of the ions near the surface. If the metal is heated, for example by-. an electric current flowing through it, some of the heat is converted into kinetic energy of the electrons. In this way some electrons gain enough energy to escape from the metal. This is what happens in Thermionic Emission. The phenomenon is analogous to the boiling off of water molecules in the form of steam from the surface of water.
The work function of the metal, W, is a measure of the amount of
energy an electron must have before it can escape from the metal
surface. It depends largely on the nature of the metal. Metals of low
work function, for example the oxides of Barium and Strontium
give off electrons at a lower temperature than metals of a high work
function such as tungsten, because the electrons do not require as
much kinetic energy.
|Homepage | Cathode Rays | Photoelectric Emission | Semiconductors |