1 - 3. Water Tension | 4. More Water Tension | 5. All About Electrolytes | 6. Hydrolysis | 7. A Cartesian Diver | 8. A Lava Lamp See how we built our Young Scientist computer. Water Experiment 5 All About Electrolytes Experiment: To show that water, with salt added, can conduct electricity.
.Materials:
You will need:
Petrie Dish Method:
What you have to do:
1)
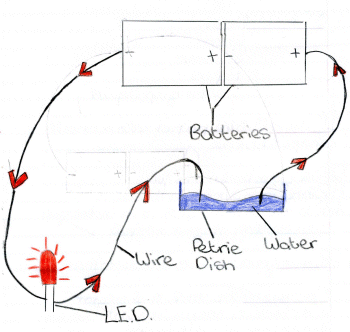
Fill the petrie dish with water to about half full.
2) Attach one end of a wire to the + terminal of the battery.
3) Attach the second wire to the - terminal of the battery.
4) Attach the third wire to the shorter terminal of the LED and place
the other end into the water in the petrie dish.
OBSERVE
5) Add salt to the water and stir until the salt is dissolved. Ideally
the water should be warm to help the salt dissolve.
Result:
The water with salt added conducted electricity and the LED lit up.
Conclusion:
Water with salt added will conduct electricity. The LED did light up a
little in ordinary water. Salty water is an electrolyte.
An electrolyte has atoms that have some
sort of electric charge - salt gives water this charge.
Warning -
Sixth |