1 - 3. Water Tension | 4. More Water Tension | 5. All About Electrolytes | 6. Hydrolysis | 7. A Cartesian Diver | 8. A Lava Lamp See how we built our Young Scientist computer. Water Experiment 6 Hydrolysis
.Materials:
You will need:
Container - margarine tub, etc. Method:
What you have to do:
1)
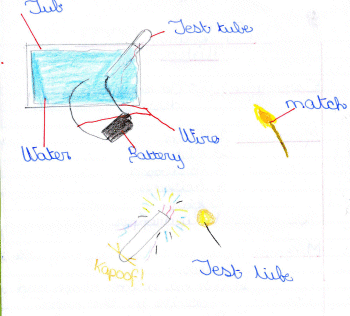
Fill the empty margarine tub with warm water.
2) Add some salt and stir well until dissolved.
3) Fill the test tube with the salty water and turn it upside down, its
side resting on the side of the tub. Blutack will help hold it steady.
4) Attach the end of one wire to the - terminal of the battery and
carefully place the other end into the mouth of the test tube.
5) Attach one end of another wire to the + terminal and place the other
end into the salty water - see diagram.
6) Leave for a few minutes.
Result:
The wire going into the test tube starts to bubble and 'fizz' as
hydrogen gas is produced. The water turns a shade of yellow.
Conclusion:
The salt turns the water into an effective electrolyte. When the circuit
is complete as both wires are immersed, one of the wires produces
bubbles - the other produces bubbles too, but far fewer. When the test
tube has completely filled with the gas, take it out of the water,
making sure no gas is allowed to escape. Light a match and see if the
gas goes POP - perfectly safe, and it proves the collected gas is
hydrogen (therefore quite explosive!)
Warning -
Sixth |

 Experiment:
To split the molecule of
water into its two parts - hydrogen and oxygen.
Experiment:
To split the molecule of
water into its two parts - hydrogen and oxygen.
