|
Chemical Reactions and Equations
An equation looks like: Reactants 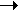 Products. Products.
Therefore, the left hand side of the equation is what we have at the beginning. The right hand side is what is left after the reaction has taken place. The reactants are used up to form the products, although sometimes some of the reactants are left over. Eg. When hydrogen reacts with oxygen to form water: H2 + ½ O2 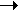 H2O. The reactants are hydrogen (H2) and oxygen (O2), while the product (only one in this case), is water (H2O). H2O. The reactants are hydrogen (H2) and oxygen (O2), while the product (only one in this case), is water (H2O).
The Mole
In short, the mole is the quantity used in chemistry to measure amounts of elements (like oxygen, hydrogen, etc.).
So in the equation: H2 + ½ O2 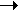 H2O, we can say that one mole of H2 reacts with a half mole of O2 to give rise to one mole of H2O. H2O, we can say that one mole of H2 reacts with a half mole of O2 to give rise to one mole of H2O.
Concentration
In chemistry, concentration is usually denoted in moles per decimetre cubed ( mol/dm3 or mol dm-3). That is how many moles of a substance would be in 1 dm3, or one litre of the sample of diluted substance.
Heats of Reaction
When a reaction takes place, it usually either gives out or takes in heat. When a reaction gives out heat, it is called a exothermic rection. When a reaction takes in heat it is called endothermic.
Catalyst
A catalyst is used in an experiment to speed up the rate of reaction. It is special, because it manages to speed up the reaction, without being used up in the process.
Site designed by Bryan Le Gear and Fintan Breen.
|