|
If a system in equilibrium is subjected to a stress (change in temperature/pressure/concentration), then the system will alter to oppose the effect of the stress
This is what happens when a reaction, at equilibrium is disturbed.
Le Chatelier's Principle allows us to predict, realistically, the effect of temperature, pressure, concentration, catalyst, etc. on the system.
To demonstrate all the following we are going to use the reaction:
N2 + 3H2 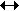 2NH3 2NH3
When nitrogen (N2) reacts with hydrogen (H2) to form ammonia (NH3)
Site designed by Bryan Le Gear and Fintan Breen.
|