|
If a moles of W react with b moles of X to give rise to c moles of Y and d moles of Z in:
aW +bX 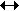 cY + dZ cY + dZ
then the Equilibrium Constant,
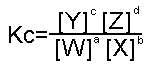
where the square brackets denote the concentration of the element inside them.
Confused? Ok, bear with us...we'll try to explain this one!
If you apply the above formula to a system in equilibrium, it has been shown to find a constant value, known as The Equilibrium Constant. This relationship is known as The Equilibrium Law
When a reaction reaches equilibrium, the above formula has been worked out to find a constant, which finds the same constant for the same reactants/products under the same conditions.
So in our the reaction:
C + H2O 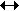 CO + H2 CO + H2
the constant would be:
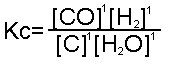
Since the number of moles of each is one, each of the concentrations is to the power of one.
Units
The units of Kc depend on the number of moles on either side of the equation. As we explained earlier concentration in measured in mol dm-3. So for the above equation:
C + H2O 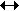 CO + H2 CO + H2
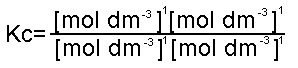
As we can see, all the units would cancel each other out. This applies for all equations when a + b = c + d in the equation:
aW +bX 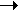 cY + dZ cY + dZ
If, as in the equation:
2NO + O2 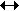 2NO2 2NO2
the number of moles on both sides of the equation is not equal, then Kc is:
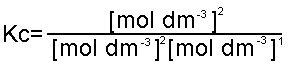
and the units are mol-1dm3
This is the same for all reversible reactions.
Site designed by Bryan Le Gear and Fintan Breen.
|