|
An equation is said to have reached equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction.
Hmmm...lets explain...
Take the example we used before:
C + H2O 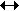 CO + H2 CO + H2
Carbon and water react, to give rise to carbon monoxide and hydrogen. But at the same time, as we explained earlier, carbon monoxide and hydrogen are reacting to give rise to carbon and water.
If we start off with only carbon and water, there is no backward equation (since there are no reactants for this equation). After a while, carbon monoxide and hydrogen have been produced, and they, in turn react together to form carbon and water.
Equilibrium is reached when carbon reacts with water to form the same ammount of carbon monoxide and hydrogen as the reaction between carbon monoxide and hydrogen produce carbon and water.
So as soon as CO and H2 are produced they react with each other, to produce C and H2O, and it all starts again. As this keeps going round and round, the actual concentration of each of the species (C, H2, CO and H2O) present is the same, once equilibrium has been reached. In other words, the concentration of any of the species is constant once equilibrium is reached.
Site designed by Bryan Le Gear and Fintan Breen.
|