|
The effect of concentration on a system in equilibrium
In the reaction:
N2 + 3H2 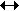 2NH3 2NH3
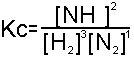
If we increase the concentration of any of the species(i.e. add more of them in), the equation will tend to shift to the other side, so as to keep Kc constant.
For instance if we add more N2 into the reaction, that makes the bottom line larger, and therefore the value for Kc would be smaller. However, Le Chatelier's Principle brings us to see that the equation 'moves' in the direction which would increase Kc, to bring it back to its 'constant' value. Therefore, adding N2 will cause to equation to move to the right, causing more ammonia (NH3) to be produced.
So adding more NH3 will cause the equation to move to the left, and more N2 and 3H2 will be produced.
Site designed by Bryan Le Gear and Fintan Breen.
|