|
The effect of pressure on a system in equilibrium (Gas reactions only)
In the reaction:
N2 + 3H2 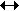 2NH3 2NH3
there are 4 (1 + 3) moles on the left hand side of the equation and 2 on the right. If we exert pressure on the reaction, according to Le Chatelier's Principle, the equation will move to minimise the effect. In other words, move to the side with the least number of moles. It will try to reduce the overcorwding of the moles in the container. So if 4 moles goes to 2 moles, like in this reaction, it will move to the side with 2, to reduce the total number of moles.
So increasing the pressure during this reaction will cause more NH3 to be produced.
Note: Increasing the pressure on a reaction with the same number of moles on either side of the equation will have no effect, as one side does not have more moles than the other.
Site designed by Bryan Le Gear and Fintan Breen.
|