|
The effect of temperature on a system in equilibrium
The reaction:
N2 + 3H2 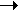 2NH3 2NH3
is exothermic, while
2NH3 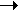 N2 + 3H2 N2 + 3H2
is endothermic.
According to Le Chatelier's Principle the equation will move to alter the stress. So an increase in temperature will favour the endothermic reaction (in this case the backward one) while a decrease will favour the exothermic one(in this case, the forward one).
Decreasing the temperature in this case caused more NH3 to be produced. Increasing the temperature will cause more N2 and H2 to be produced.
Site designed by Bryan Le Gear and Fintan Breen.
|